Abstract
Background: Single cases of aggressive B-cell non-Hodgkin lymphomas upon treatment with the Janus kinase 1/2 (JAK1/2) inhibitor ruxolitinib in patients with myeloproliferative disorders (MPD) have been reported (Pardanani, Mayo Clin.Proc. 2011 ; Bhatt VR et al. J Natl Compr Canc Netw . 2015;13(3):281-28; Palmason et al. BMC Hematology 2015). However, incidence and pathogenesis remain enigmatic.
Methods: We investigated 626 patients with myeloproliferative neoplasms diagnosed at the Medical University of Vienna between 1997 and 2016. Of these, 69 patients with primary or secondary myelofibrosis were treated with JAK2 -inhibitors since 2009. Data on secondary malignancies were retrieved from our MPN database. Detailed clinical, pathologic and genetic data were obtained in patients developing lymphoma. These observations were recapitulated in a Stat1 knock-out mouse model (Stat1-/- mice).
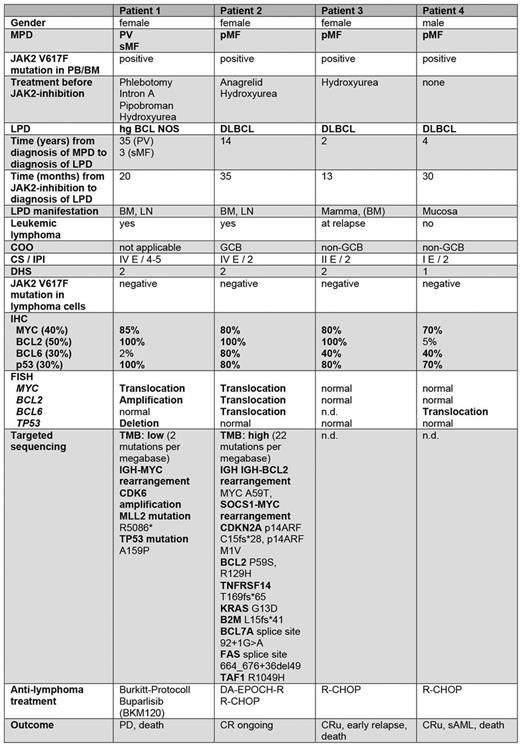
Results: B-cell lymphomas developed in 4 out of 69 patients (5.8%) upon ruxolitinib treatment compared to only 2 of 557 (0.36%) in the control group (16-fold increased risk, p=0.0017). The median time to lymphoma diagnosis was 25 months after ruxolitinib initiation (Table 1). Two lymphoma patients had been pre-treated with alkylating agents before ruxolitinib, one with hydroxyurea, the JAK -inhibitor fedratinib and ruxolitinib, and one received ruxolitinib only. All lymphomas were of aggressive CD19+ B-cell type (3 DLBCL, 1 HG BCL NOS) and showed distinct features with extranodal involvement and/or bone marrow infiltration (3/4), IPI of 2 or greater, and high MYC, and TP53 expression. Genetic analysis revealed abnormalities in MYC, BCL2, BCL6 or TP53 genes (Table 1). Targeted sequencing in 2 patients showed inactivating mutations in the B2M, CDKN2A, or TP53 genes. No JAK2 -mutation was found in the lymphomas. Three of four patients responded to anti-lymphoma therapy, but 3 patients eventually died from lymphoma or sAML. Of note, the clinical, and pathogenetic features closely resembled those of the published cases and 2 cases seen at a different institution (Paris, Hospital Saint-Louis).
Mice deficient for the JAK kinase target STAT1 mirrored the course of the human disease: 16 of 24 Stat1-/- mice developed myeloid hyperplasia with splenomegaly and a massive expansion of myeloid blasts in bone marrow and peripheral blood. Lowering the load of myeloid blasts by ATRA or ruxolitinib led to B-cell proliferation. Transplantation of bone marrow from diseased Stat1-/- mice resulted in aggressive B-cell lymphomas displaying strikingly similar features to the human lymphomas with upregulation of c-Myc and Bcl-2 and downregulation of B2m (ß2-microglobulin) and Cdkn2a . STAT3 and STAT5 were not activated in these B-cell lymphomas indicating that the JAK/STAT pathway is dispensable for B cell transformation. B cells from diseased Stat1-/- mice gave rise to stable growth-factor independent cell lines, underlining their high grade of transformation.
Conclusion. Inhibition of the JAK/STAT1 pathway in myeloproliferative diseases appears to provoke the development of a distinct type of aggressive B-cell lymphoma. In myelofibrosis treated with a JAK2 inhibitor 5 to 6% of patients are affected. Further investigations to identify potential risk factors are ongoing.
Sperr: Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Phadia: Research Funding; Meda: Research Funding; Teva: Honoraria; Novartis: Other: Register. Staber: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Abbie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; MSD: Honoraria; Amgen: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Valent: Blueprint: Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Incyte: Honoraria; BMS: Honoraria; Ariad: Honoraria, Research Funding; Pfizer: Honoraria; Teva: Honoraria; Deciphera: Honoraria, Research Funding. Hoermann: Gilead: Honoraria, Research Funding; Ariad: Honoraria; Amgen: Honoraria; Novartis: Honoraria. Kiladjian: Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Krauth: Novartis: Honoraria; Celgene: Honoraria; AOP Orphan Pharmaceuticals AG: Honoraria; BMS: Honoraria; Takeda: Honoraria; Janssen Cilag: Honoraria; Amgen: Honoraria. Jaeger: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gisslinger: PharmaEssentia: Consultancy, Honoraria; AOP Orphan Pharmaceuticals AG: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Shire: Honoraria; Janssen Cilag: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.